NextDose: A web-based Bayesian dose forecasting
tool
Login†††
Last updated 16 June 2024
Concentration Guided Dosing + Target Concentration
Intervention
NextDose
was developed initially for use in Auckland to help clinicians and laboratory staff
make the best use of concentration and biomarker measurements for dose individualisation.
It is a collaborative
web-based tool developed to meet the clinical need for improved patient dosing.
It is freely available in order to promote more
widespread use of concentration guided dose individualization using a target
concentration intervention approach.
Concentration
guided using with Bayesian forecasting has been proven to save lives. Evans et
al. demonstrated a larger increase in survival for childhood acute
lymphoblastic leukaemia, by individualising
methotrexate dose, than any other single drug treatment (Evans, Relling et al. 1998). Despite its striking improvement on
5-year survival, this method has been hardly used because of the difficulties
of access to, and usability of, Bayesian forecasting
software.
Dose individualisation has also been shown to increase survival
after busulfan conditioning by maintaining population concentrations within a
narrow safe and effective range (Bleyzac, Souillet et al. 2001; Bolinger, Zangwill et al. 2001;
Booth, Rahman et al. 2007; Bartelink, Bredius et al. 2009; Abbasi, Vadnais et al. 2011; Hempel
and Trame 2011; Long-Boyle et al. 2015).
Warfarin
dose predictions using NextDose with INR measurements have been shown to be
accurate across a wider range of daily doses (Holford, Ma, Tsuji 2018) than
other Bayesian algorithms (Saffian et al. 2016). This warfarin PKPD model has
been implemented in NextDose and shown to improve the time within the
acceptable range and reduce bleeding (Xue, Ma et al.
2024).
The
Need
In January 2012, busulfan
analysis and reporting with NextDose was demonstrated to the Clinical Director
and lab technicians of LabPlus as well as
pharmacists, nurses, haematologists and oncologists
associated with the Auckland Starship Blood and Cancer Centre. The opportunity
for improved clinical care by using Bayesian forecasting for dosing of busulfan
was demonstrated and accepted.
These Bayesian forecasting
methods have not been available, in a clinically
useful form, in Auckland until 2012 when it was decided that NextDose should be
adopted to help busulfan dosing using.
Technology
NextDose has been designed
around a database to provide security and transportability. This approach
allows patient data to be stored remotely and allows collaboration between members
of the clinical and laboratory team. The framework is extensible to other
medicines, with their associated observation and reporting types. NextDose
consists of three software abstraction layers to provide a clear separation
between the user interface, model controllers and the modelling software. This
modular approach allows the use of different modelling software via the same
intuitive interface, which should make these tools more accessible and useable in a clinical environment.
Evolution
The original model used for
busulfan was based upon a modification of the PK model developed by FDA (Booth,
Rahman et al. 2007). The NextDose modification employed a maturation function
to help predict doses in infants. In 2014 the model was updated based on a
large data set collected in infants, children and adults (McCune et al. 2014).
The model was further refined in 2017 to improve the empirical prediction of
clearance changes with time.
Graphical
View of Busulfan Dose Individualisation
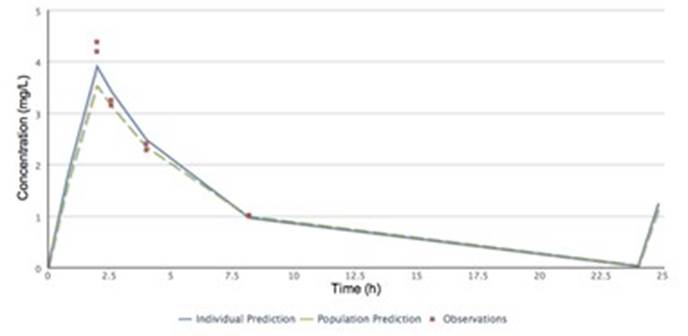
Concentration-time course
of busulfan predicted with NextDose
The observations (red) show
duplicate concentration measurements. The population prediction (green) shows
the profile predicted based on the patientís age, weight and other
characteristics. The individual prediction is based on a weighted combination
of the population prediction, and observations, and
represents the best estimation of the patientís actual concentration-time
profile. If there is a delay between the end of infusion and ideal peak sample
time, the Bayesian method allows extrapolation to the infusion end time, which
is vital for area-under-curve based dosing targets.
References
Abbasi, N., B. Vadnais, et al. (2011). "Pharmacogenetics of Intravenous and Oral Busulfan in
Hematopoietic Cell Transplant Recipients." The Journal of Clinical
Pharmacology 51(10): 1429-1438.
Bartelink, I. H., R. G. M. Bredius, et al. (2009). "Association between busulfan
exposure and outcome in children receiving intravenous busulfan before
hematologic stem cell transplantation." Biology of Blood and Marrow
Transplantation 15(2): 231-241.
Bleyzac, N., G. Souillet,
et al. (2001). "Improved clinical outcome of paediatric
bone marrow recipients using a test dose and Bayesian pharmacokinetic
individualization of busulfan dosage regimens." Bone Marrow Transplant
28(8): 743-751.
Bolinger, A. M., A. B.
Zangwill, et al. (2001). "Target dose adjustment of busulfan in pediatric
patients undergoing bone marrow transplantation." Bone Marrow Transplant
28(11): 1013-1018.
Booth, B. P., A. Rahman, et
al. (2007). "Population pharmacokinetic-based dosing of intravenous
busulfan in pediatric patients." J Clin Pharmacol 47(1):
101-111.
Evans, W. E., M. V. Relling, et al. (1998). "Conventional compared with individualized chemotherapy for
childhood acute lymphoblastic leukemia." New England Journal of Medicine
338(8): 499-505.
Hempel, G. and M. N. Trame
(2011). "Therapeutic drug monitoring of busulfan." Clin Chem 57(4):
643-644.
Holford N, Ma G, Tsuji Y.
Using biomarkers to predict the target dose of warfarin and linezolid. PAGE.
2018;27[www.page-meeting.org/?abstract=8562].
Long-Boyle JR, Savic R, Yan
S, Bartelink I, Musick L, French D, et al. Population
pharmacokinetics of busulfan in pediatric and young adult patients undergoing
hematopoietic cell transplant: a model-based dosing algorithm for personalized
therapy and implementation into routine clinical use. Ther Drug Monit.
2015;37(2):236-45.
McCune JS, Bemer MJ,
Barrett JS, Scott Baker K, Gamis AS, Holford NHG.
Busulfan in Infant to Adult Hematopoietic Cell Transplant Recipients: A
Population Pharmacokinetic Model for Initial and Bayesian Dose Personalization.
Clin Cancer Res. 2014;20(3):754-63.
Saffian SM, Duffull SB,
Roberts RL, Tait RC, Black L, Lund KA, et al. Influence of Genotype on Warfarin
Maintenance Dose Predictions Produced Using a Bayesian Dose Individualization
Tool. Ther Drug Monit. 2016;38(6):677-83.
Xue, L., G. Ma, N. Holford,
Q. Qin, Y. Ding, J. A. Hannam, X. Ding, H. Fan, Z. Ji, B. Yang, H. Shen, Z.
Shen and L. Miao (2024). "A Randomized Trial Comparing Standard of Care to
Bayesian Warfarin Dose Individualization." Clinical Pharmacology &
Therapeutics 115(6): 1316-1325.
Copyright All rights
reserved | Developed by Sam Holford & Nick Holford 2012-2024