NextDose: A web-based Bayesian dose
forecasting tool
Posaconazole
Posaconazole is
an antifungal agent that is administered orally. It is available as a tablet
and liquid suspension. The suspension has a lower relative bioavailability
and the extent of absorption is decreased by concomitant use of proton pump
inhibitors and by diarrhoea.
The population
pharmacokinetics of posaconazole have been described in children by (Boonsathorn, Cheng et al. 2019). Theory based
allometry and standardization to body mass of 70 kg allows extrapolation to
adults.
Target Concentration
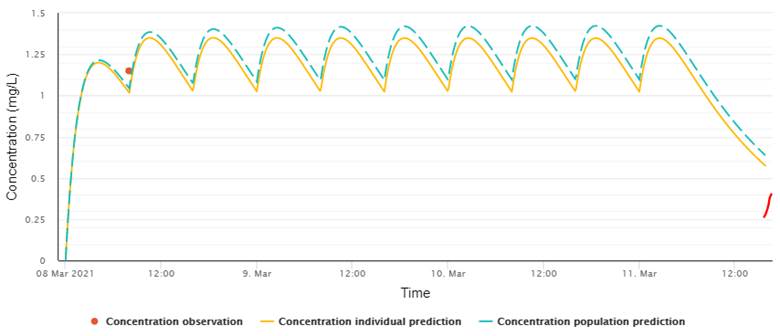
The recommended target
concentration for treatment is an average steady state concentration of 1.25
mg/L or a steady state trough of 1 mg/L with an 8 h dosing interval. A lower
steady state trough concentration of 0.7 mg/L is recommended for prophylaxis (Ashbee, Barnes
et al. 2014). The average steady state treatment
target comes from an exploratory analysis of the association between exposure
metrics and clinical response (Figure 1) (Walsh, Raad et al. 2007).
Figure 1 Exposure response (Walsh, Raad et al. 2007)
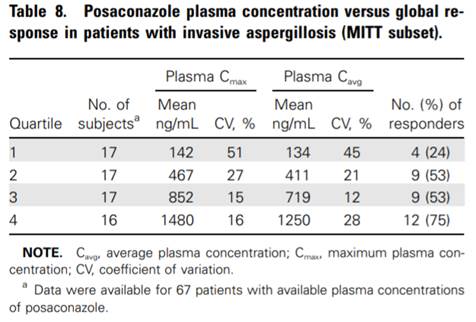
Figure 1 Posaconazole oral tablet
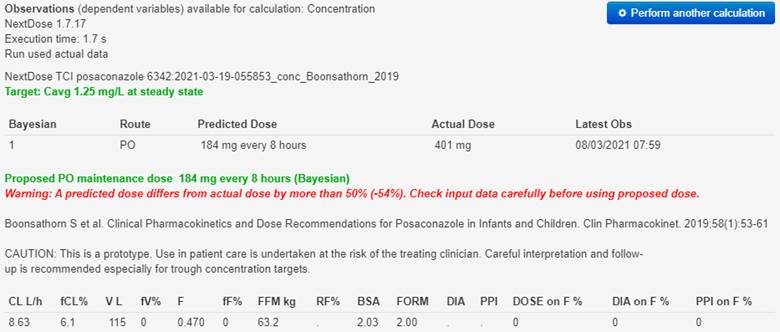
There is no effect of diarrhoea (DIA), proton pump inhibitors (PPI) on
bioavailability (F).
References
Ashbee, H. R., R. A. Barnes, E. M. Johnson, M. D. Richardson, R. Gorton
and W. W. Hope (2014). "Therapeutic drug monitoring (TDM) of antifungal
agents: guidelines from the British Society for Medical Mycology." J
Antimicrob Chemother 69(5): 1162-1176.
Boonsathorn, S., I. Cheng,
F. Kloprogge, C. Alonso, C. Lee, B. Doncheva, J. Booth, R. Chiesa, A. Irwin and
J. F. Standing (2019). "Clinical Pharmacokinetics and Dose Recommendations
for Posaconazole in Infants and Children." Clin Pharmacokinet 58(1):
53-61.
Walsh, T. J., I. Raad, T.
F. Patterson, P. Chandrasekar, G. R. Donowitz, R. Graybill, R. E. Greene, R.
Hachem, S. Hadley, R. Herbrecht, A. Langston, A. Louie, P. Ribaud, B. H. Segal,
D. A. Stevens, J.-A. H. van Burik, C. S. White, G. Corcoran, J. Gogate, G.
Krishna, L. Pedicone, C. Hardalo and J. R. Perfect (2007). "Treatment of
Invasive Aspergillosis with Posaconazole in Patients Who Are Refractory to or
Intolerant of Conventional Therapy: An Externally Controlled Trial." Clinical
Infectious Diseases 44(1): 2-12.
Copyright All rights reserved | Developed by Sam Holford & Nick
Holford 2012-2021